I have a disease called hereditary hemochromotosis, abbreviated HH for short. However, I know next to nothing about it despite being diagnosed nearly a year ago. This is my fault, since I've left it up to the public health service in Ireland to look after me, and really I'm not an urgent case. However, just because I am not dying doesn't mean my life is not adversely affected. In actual fact, I don't know how much my life is being adversely affected, so I am going to use my blog to teach myself and others about this disease. It's also a generally interesting insight into the genetics of an interesting metabolic disease.
Hemochromotosis is a disease that results in abnormally high levels of iron in the blood. It is a genetic disorder, meaning it is not acquired as a result of infection from bacteria or viruses for example, and is not transmissible by person to person contact. To date there is no cure, only management of the symptoms.
WHAT HAS IRON EVER DONE FOR US?
Iron is a normal part of our diet, and is required for numerous aspects of healthy metabolism. For example, blood cells use iron to transport oxygen from the lungs to the rest of the body. A lack of iron in our diet, anemia, is also a problem, and can lead to a range of symptoms such as fatigue, and dizziness. Commonly ingested foods such as breads and breakfast cereals are fortified with iron to help prevent anemia.
However, excess levels of iron in the blood is also a problem, and is can lead to fatigue, lack of concentration, hair loss, mood disorder, liver failure, heart failure, and abormal pituitary gland function (associated with the release of testosterone, growth hormone, and sperm production in men).
WHAT HAS IRON EVER DONE FOR US?
Iron is a normal part of our diet, and is required for numerous aspects of healthy metabolism. For example, blood cells use iron to transport oxygen from the lungs to the rest of the body. A lack of iron in our diet, anemia, is also a problem, and can lead to a range of symptoms such as fatigue, and dizziness. Commonly ingested foods such as breads and breakfast cereals are fortified with iron to help prevent anemia.
 |
| The wide ranging symptoms of anemia |
To understand hemochromotosis requires an understanding of both genetics and protein biochemistry. Consequently I'm going to separate this into two parts, one dealing with the genetics of the disease, the other dealing with the biochemical manifestation of the disease. Both are interesting in their own right, and both are important to understand, because an understanding of the genetics allows you to predict how your children will be affected, and an understanding of the biochemistry allows you to make some lifestyle changes that might make a difference in how the disease progresses.
THE GENETICS OF HEMOCHROMOTOSIS
A typical educated layman is often precluded from understanding relevant scientific concepts, especially when it comes to their health. Science, and medicine are packed with acronyms, and unique meanings for words you thought you understood. Many people will be familiar with words such as gene, genome, DNA, chromosome, and inherited. Some may have heard of more specific terms such as autosomal, recessive, and dominant. My previous blogpost, found here covers the basics of what a gene is, and how genes are inherited, so you may want to read that before going any further. It will guide you through this interesting maze of terminology.
TYPES OF HEMOCHROMOSTOSIS
There are three types of HH simply called type 1, 2, and 3. These are caused by mutations in one or more of 5 genes located on 5 different chromosomes.
There are three types of HH simply called type 1, 2, and 3. These are caused by mutations in one or more of 5 genes located on 5 different chromosomes.
- The HAMP gene (Hepcidin Antimicrobial Peptide), located on chromosome 19
- The HFE gene (Hemochromatosis), located on chromosome 6
- The HFE2 gene (Hemochromatosis 2), located on chromosome 1
- The SLC40A1 gene (solute carrier family 40), located on chromosome 2
- The TFR2 gene (Transferrin Receptor 2), located on chromosome 7
There are actually two subtypes of type 2 hemochromotosis, called type 2A and type 2B. This is to represent the fact that they arise on different genes. Type 2A is the result of a mutation in the HFE2 gene, while type 2B is the result of a mutation in the HAMP gene. However, the majority of people with HH carry a mutation in the HFE gene.
AUTOSOMAL RECESSIVE DISORDER
Hemochromostosis is what's called autosomal recessive disorder. This means you must have two copies of the mutated gene to actually have the disease. The only way this can occur is if both parents are carriers of the disease (heterozygous for the mutated HFE gene, or actually have the disease (homozygous for the mutated HFE gene). The punnett squares below show the possible genetic crosses considering instance where (a) both the mother and father are heterozygotic, (b), the mother is heterozygotic, and the father is homozygous for hemochromotosis and (c) both parents are homozygous for the disease.
If we assume "H" and "h" represent any one of the five genes for hemochromotosis, and that "H" is the a normal working version of the gene, while "h" is a mutated broken copy of the gene, then someone with hemochromatosis has the "hh" set of genes. You can see from (a)-(c) above that even if both parents are heterozygous for hemochromostosis, they still have a 1:4 chance of producing offspring that will have the disease.
PREVALANCE OF AUTOSOMAL RECESSIVE DISORDER
Autosomal recessive disorders tend to result in isolated populations. Think of it as having just one copy of an important textbook that is newly transcribed and past down each generation, for many generations. Over time, no matter how hard people try, flaws in the transcription process will arise, and they will be propagated from generation to generation. Over time, you end up with a flawed version of the textbook. However, if there was another group of people who also had the same textbook, and were also constantly transcribing, it is unlikely they will have made exactly the same mistakes. So merging the two textbooks together will result in less flawed version of the textbook. When you look at the prevalence of HH in Europe you can immediately see that Ireland has the highest instance at between 10-12.8% of the population!
 |
| Taken from "EASL clinical practice guidelines for HFE hemochromatosis" Journal of Hepatology 2010 vol. 53 Pg3–22 |
This is true of other autosomal recessive disorders, for example there are twice as many cystic fibrosis suffers in Ireland as there are anywhere else in Europe, 25 per 100,000 in Ireland compared to the next highest, Belgium at 11 per 100,000. Interestingly, if there is a sustained influx of new genetic material from outside of Ireland then the prevalence of all autosomal recessive diseases would decline.
THE HEMOCHROMOTOSIS GENES & GENETIC MUTATIONS
As mentioned, hemochromotosis can result from mutation in any one of 5 genes. The term mutation sounds quite scary, but geneticists use this word to describe a chemical change in the composition of DNA. For example, the genetic sequence presented in the last blog post was as follows,
Remember, each set of letters is A-T, G-C, is called a base pair. A genetic mutation in this sequence would mean one of the four letters of DNA being replaced with any one of the other three letters of DNA. So, "T" might be replaced with "G" in just one location in the above sequence, (below) and this can be enough to completely destroy the functionality of this gene!
The biology behind how such a small change can result in such a dramatic effect is both fascinating and complex, and I will post an entry on that some other time. For the moment, all you need to know is that such mutations are described as point mutations, and they are pretty common. They arise from environmental effects such as UV radiation from the sun, but also from normal biological events such as cell replication. There's no avoiding it, they will occur, it's just a case of where exactly, and what effect they will have. Some point mutations will result in no difference what-so-ever to the function of the gene.
As mentioned, hemochromotosis can result from mutation in any one of 5 genes. The term mutation sounds quite scary, but geneticists use this word to describe a chemical change in the composition of DNA. For example, the genetic sequence presented in the last blog post was as follows,
Remember, each set of letters is A-T, G-C, is called a base pair. A genetic mutation in this sequence would mean one of the four letters of DNA being replaced with any one of the other three letters of DNA. So, "T" might be replaced with "G" in just one location in the above sequence, (below) and this can be enough to completely destroy the functionality of this gene!
The biology behind how such a small change can result in such a dramatic effect is both fascinating and complex, and I will post an entry on that some other time. For the moment, all you need to know is that such mutations are described as point mutations, and they are pretty common. They arise from environmental effects such as UV radiation from the sun, but also from normal biological events such as cell replication. There's no avoiding it, they will occur, it's just a case of where exactly, and what effect they will have. Some point mutations will result in no difference what-so-ever to the function of the gene.
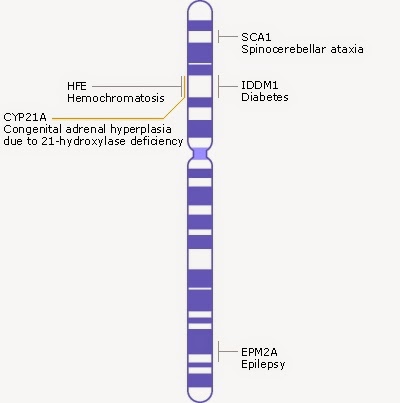 |
| Schematic of chromosome 6, taken from http://www.ncbi.nlm.nih.gov/books/NBK22266/#A278 |
THE HFE GENE
Chromosomes are depicted as shown in the image on the right. The blue bands represent clusters of genes. Chromosome six is composed of 170 million base pairs and contains about 2,000 genes. The HFE gene is just one of these genes, and is 8,000 base pairs long. There are 20 different locations along this 8,000 base pair region which will result in hemochromotosis. However, there are two locations in particular where mutations occur quite frequently, and they result in two changes in the HFE protein. These mutations are called C282Y, and H68D.
There are at least 52 additional point mutations across the HAMP, HFE2, SLC40A1, and TFR2 genes that can all result in hemochromtosis.
MEDICAL RELEVANCE
I mentioned in the beginning that I have hemochromotosis. However, this may not be entirely accurate. My situation is that I was screened for HH on the basis of a blood test which showed elevated iron levels. However, the genetic test showed an unusual result. Most people with HH are homozygous in that they have two copies of the defective HFE gene. My results came back as being heterozygous, with one copy of my HFE gene having both the C282Y, and H68D mutation, which my doctor says should not result in any symptoms. However, I do have at least some of the symptoms, and I would like to rule out HH as one of the causing factors. Until I started researching this disease I was unaware there were so many genes and point mutations that could result in its development. Personally, I find it interesting, but I would also like to know that my doctors have considered the possibility that I also carry a defective copy of one of the other HH genes. However, I think I am correct in saying that the probability of me having another defective copy of a mutated gene which relates to HH, but is not the HFE gene are reasonably slim due to something called conjunction fallacy, see here.
CONCLUSION
So, you now understand the basics of hemochromotosis. There will undoubtedly be much more for me to discover about this disease, and as mentioned, I plan to provide additional posts that go into details on the biochemistry of the disease. Each of these defective genes make a unique protein that misbehaves in a unique way which will very exciting to learn more about. I appreciate this is a very technical blog post, so if there were things you did not understand it's because I have not explained them well. If this is the case, feel free to leave a comment below, and I will happily review and re-write to make it better.
USEFUL MATERIAL
Original research paper Hemochromatosis types 1, 2, and 3
I mentioned in the beginning that I have hemochromotosis. However, this may not be entirely accurate. My situation is that I was screened for HH on the basis of a blood test which showed elevated iron levels. However, the genetic test showed an unusual result. Most people with HH are homozygous in that they have two copies of the defective HFE gene. My results came back as being heterozygous, with one copy of my HFE gene having both the C282Y, and H68D mutation, which my doctor says should not result in any symptoms. However, I do have at least some of the symptoms, and I would like to rule out HH as one of the causing factors. Until I started researching this disease I was unaware there were so many genes and point mutations that could result in its development. Personally, I find it interesting, but I would also like to know that my doctors have considered the possibility that I also carry a defective copy of one of the other HH genes. However, I think I am correct in saying that the probability of me having another defective copy of a mutated gene which relates to HH, but is not the HFE gene are reasonably slim due to something called conjunction fallacy, see here.
CONCLUSION
So, you now understand the basics of hemochromotosis. There will undoubtedly be much more for me to discover about this disease, and as mentioned, I plan to provide additional posts that go into details on the biochemistry of the disease. Each of these defective genes make a unique protein that misbehaves in a unique way which will very exciting to learn more about. I appreciate this is a very technical blog post, so if there were things you did not understand it's because I have not explained them well. If this is the case, feel free to leave a comment below, and I will happily review and re-write to make it better.
USEFUL MATERIAL
Original research paper Hemochromatosis types 1, 2, and 3


Hi Dr Who!
ReplyDeleteI came across this post in researching HH for myself. I also have one of each of the two genes, C282Y and H68D. I just wanted to clarify what your doctor said: " My results came back as being heterozygous, with one copy of my HFE gene having both the C282Y, and H68D mutation, which my doctor says should not result in any symptoms."
This is an old post but...isn't your doctor is wrong about that? Having both mutations mean you have a moderate risk of iron overload, and that you have HH, so you would be having symptoms, just not as seriously as having both C282Y mutations. Thought that was interesting!
I think there is still so much left unknown about HH!